iNDX.AI Recognized on Pharma Tech Outlook’s list of Top 10 Analytics Solution Providers – 2020
iNDX.AI Recognized on Pharma Tech Outlook’s list of Top 10 Analytics Solution Providers – 2020 Cupertino, CA, Novemb
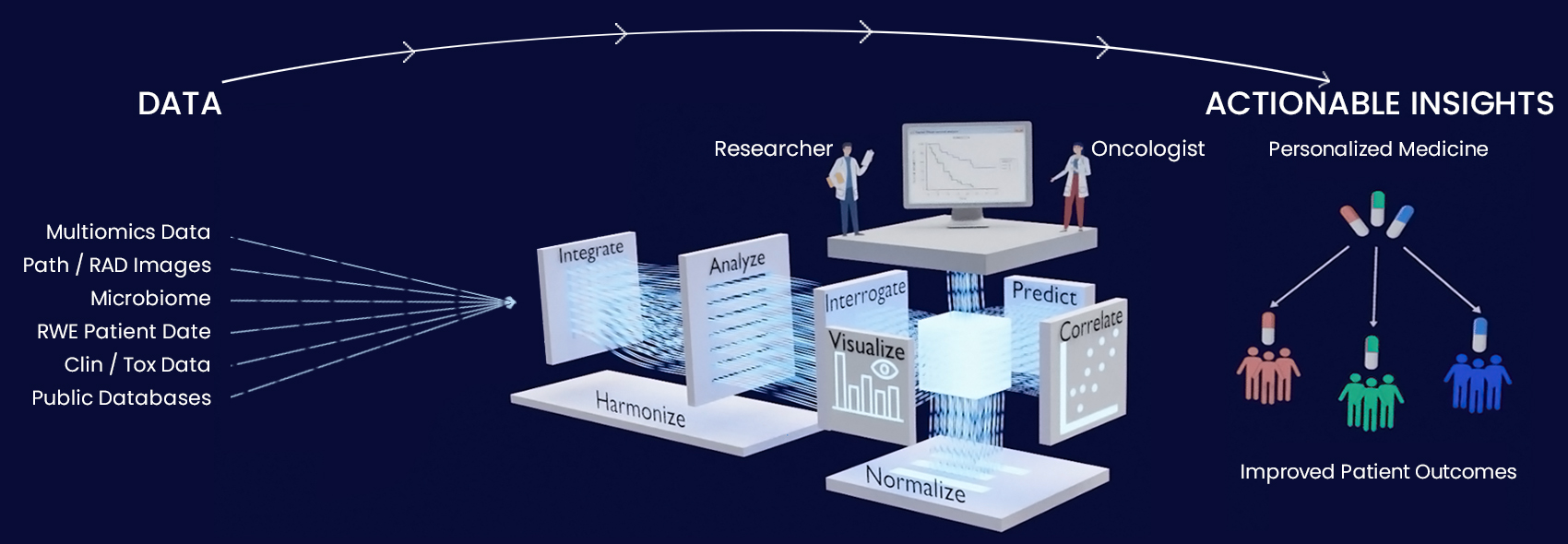
AI Enabled Biomarker Discovery Platform Accelerating Translational Research
Accelerating Translational Research with Deep and Dense Data Analytics
Comprehensive data analytics platform integrating ecosystem to accelerate biomarker-based clinical trials
iCore™
Integrated Informatics Platform for Accelerating Biomarker-Rich Clinical Trials
Patients
Clinician
Study Director
Researcher
Bioinformatician
iCore Cloud

Features
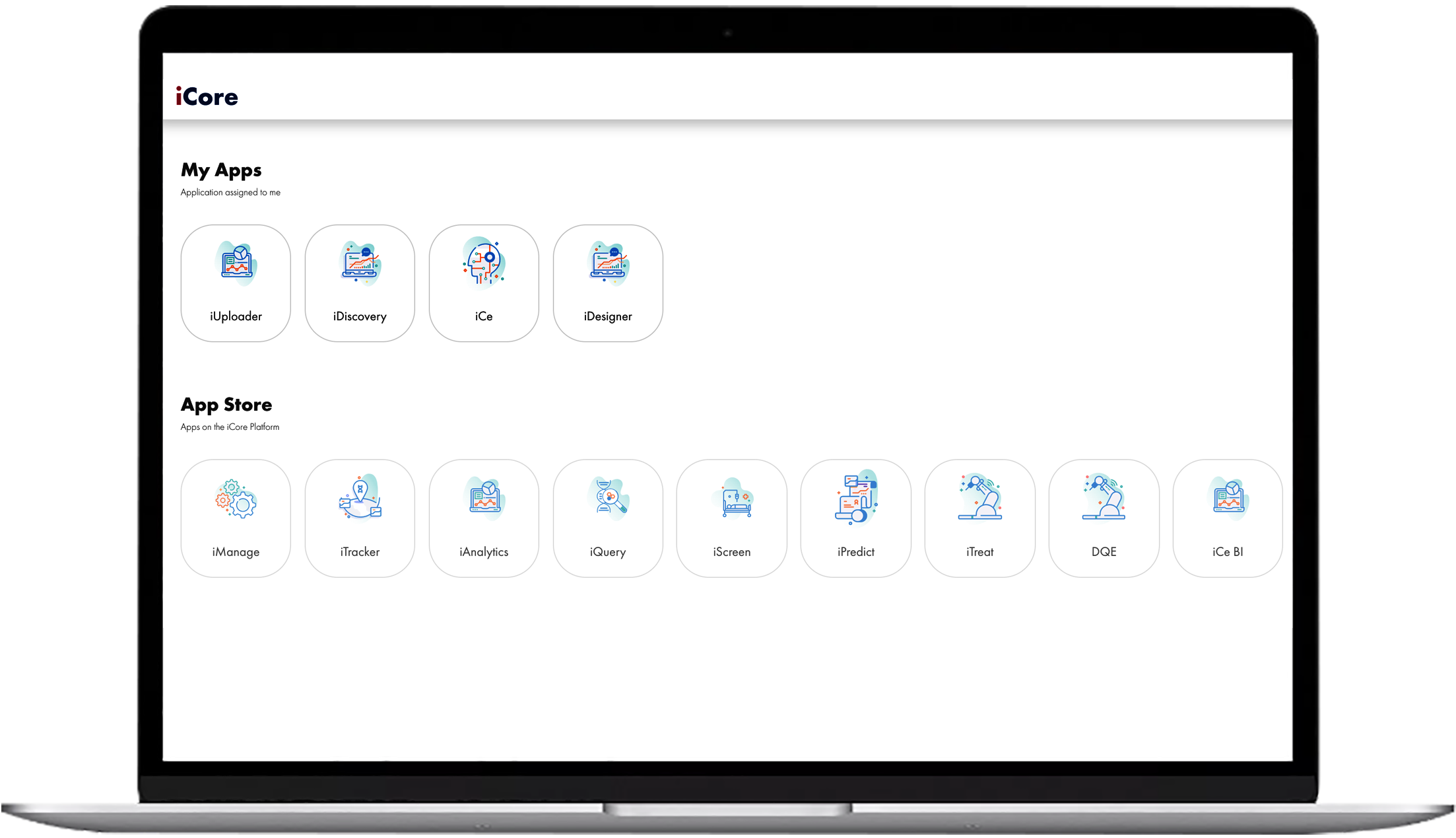
Modern, Scalable & Secure
GDPR, 21 CRP Part 11, HIPAA and GxP
High security with advanced encryption
Easy to integrate (Open API)
Easy to customize
Real-time data access (API first approach)
A visual experience you’ll fall in love with
Values
A revolutionary informatics platform offering an integrated ecosystem for managing data-intensive and biomarker-rich precision medicine clinical trial


• Provide Analytical Services
• Automated Primary & Secondary Analysis
• Seamless Collaboration

• Save Cost on Data Management & Analysis
• Identify Biomarker Signature
• Find out the Right Patients for the Trial
• Improve the Final Clinical Trial Outcome

• Ease of Uploading Data
• Giving access to the Data to Customer
• Reduce Time for Biomarker Data Management

• Holistic View of entire Clinical Study
• Refer patients to trials
• Connect with Care Teams
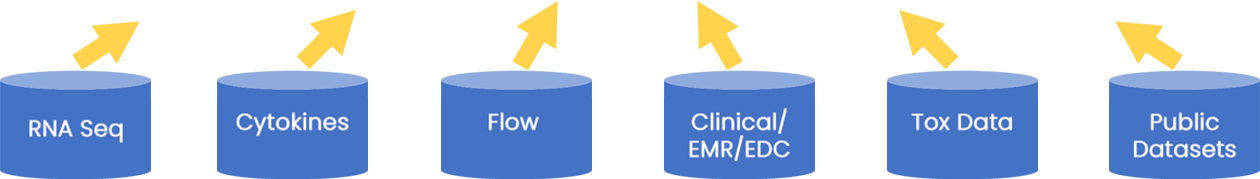
Genomics
Cellular
Cytokine
Clinical
Imaging
Lab Data

Accelerating Translational Research with Deep and Dense Data Analytics
News & Articles
iNDX.AI Recognized on Pharma Tech Outlook’s list of Top 10 Analytics Solution Providers – 2020 Cupertino, CA, Novemb
iNDX.Ai announces 21 CFR part 11 compliance of its flagship product iCore Cupertino, CA – July 7, 2019 In a major regu
iNDX.Ai Announces iCore Compliance with HIPAA Sep 16, 2019 Compliance with HIPAA. Cupertino, CA – May 19, 2019, iNDX T
iNDX Technology,Inc recognized as “Top 10 Bioinformatics Solution Providers – 2019” by CIO Applicant iNDX.Ai, a Bi
DMN Newswire: Leading Life Sciences and IT Companies Join End-pandemic National Data Consortium Industries Answer Call-t
iNDX.AI’s iCore Data Analytics Platform receives GXP compliant certification Cupertino, CA, February 12, 2021. iNDX.Ai
Collaborative case study for academic center Jaydeep Adhikari – Mar 23 The AAPM Annual Meeting 2021 brought togeth
Host‑dependent molecular factors mediating SARS‑CoV‑2 infection Gowhar Shafi·Shruti Desai·Krithika Srinivasan·A
OUR PARTNERS
Testimonials
What Our Customers Are Saying
We keep our clients at the top of our priority list. And that’s how we’ve managed to build ahappy customer base.
Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy
Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy
Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy text of printing and typesetting industry lorem ipsum been Lorem ipsum is simply dummy
Years Of
Experience
Business
Partners
Product
Installed
Copyright 2024 iNDX.Ai. All rights reserved.

____________________________________________________________________________________________
Coming Soon

____________________________________________________________________________________________
Coming Soon

____________________________________________________________________________________________
Denis earned his B.S. and M.S. in mechanical engineering from MIT and his Ph.D. in management (computer science minor) from Stanford. In 1979 he obtained his CPA and left an academic career that featured teaching positions in Canada, the University of Hawaii, and Stanford.
In his first project, he wrote the software and ran a company that sold the first spelling checker for microcomputers. Then in 1983, he co-founded a software company now known as Symantec Corporation, the fourth largest software company in the world in 2007. He spent five years at Symantec, serving as VP of R&D for development of the initial product, Q&A. This productivity software package won many product-of-the year awards and eventually had retails sales of over half a billion dollars.
Since 1989, he has served variously as early board member, founder, software designer/developer in ten other startups, two of which had IPO’s — NEOF (online medical supply and equipment marketplace) and VSNR (hardware/software office imaging solutions, now merged with Nuance). Financial Engines (Internet based retirement investments) and Claria (Internet advertising) each achieved profitability and annual revenues in excess of $80 million. C Level Design (C language translated into ASIC hardware designs) and WealthCycle (Internet based investment advise) had successful product development but hasty exit sales to larger entities after the bubble burst of 2001.
Since 2004, he has been interested in bioengineering, particularly in the area of drug discovery and has shifted new portfolio investments and startup energies to the life sciences.
He has been a member of the Band of Angels since 1997, serving on deal screening committees and making about 12 private investments.

Chair- Department of Pathology, Baystate Health, Medical Director- Baystate Reference Laboratories, Professor and Deputy Chairman- Department of Anatomic & Clinical Pathology, Tufts University School of Medicine
____________________________________________________________________________________________
At Baystate Health, Dr. Friedberg leads a hybrid academic/private practice group of 21 pathologists and a regional reference laboratory providing exclusive diagnostic services to 5 hospitals, numerous nursing homes, and more than 3300 providers across Massachusetts. He is also Professor and Deputy Chairman of the Department of Pathology, Tufts University School of Medicine. Dr. Friedberg holds a Bachelor Science (BS) with Honors from Stanford University, an MD from Duke University, a PhD in coagulation biochemistry from Duke University, and a Master’s (SM) in Health Care Management from Harvard University. He is a Certified Physician Executive (CPE) by the American College of Physician Executives. Over the past 20 years, he has also served on numerous committees and councils for the College of American Pathologists (CAP), including the Government & Professional Affairs, Accreditation, Quality Practices, Technology Assessment, Transformation, Finance, & Transfusion Medicine. In 2007 and again in 2010, he was elected by the CAP membership to serve on the CAP Board of Governors. In 2013, the CAP membership elected him to serve as CAP President-Elect and will be CAP President from 2015-17

____________________________________________________________________________________________
Carol has over 25 years of executive management experience in the diagnostic, medical device, and biotechnology industry. Carol joined Caprion in January 2014 as the Chief Business Officer and Sr. Vice President responsible for the commercial strategy and activities related to business development, marketing, product development, and customer service.
Prior to joining Caprion, Carol served as the Senior Vice President and General Manager for Asuragen’s Genomic Services and CLIA Laboratory Division. From 2002 to 2007 Carol was a member of the senior management team at Ciphergen Biosystems Inc., a proteomic equipment and pharmacoproteomic research company. At Ciphergen she was responsible for diagnostic business development, sales and marketing, and assessing new potential diagnostic discoveries viable for commercialization. Carol served as the Vice President of Sales/Marketing for Dynacare Laboratories for three years where she was instrumental in expanding Dynacare’s market share to a national ranking through acquisitions, strategic partnerships with hospitals, and organic sales growth nationwide. Carol started her diagnostic career at Roche Biomedical Laboratories where she was one of the top fifty General Managers from 1986 to 1996.
Carol received her Bachelor of Science Degree from Texas A&M University where she was a four year Letterman on the Women’s Golf Team. She received her MBA-Information Technology Degree from University of Dallas in 2004.

____________________________________________________________________________________________
Coming Soon

____________________________________________________________________________________________
Dr. Gowhar Shafi has done his Doctorate in Medical Genetics from the Institute of Genetics & Hospital for Genetic Diseases & Dr. NTR University of Health Sciences, Hyderabad, India. He completed his postdoctoral research in Computational Medicine from Karolinska Institute, Stockholm, Sweden. Dr. Gowhar held the positions as Head Genomics/Bioinfomatics and Chief Scientific Officer at Positive Bioscience, India between 2014 and 2018. Prior to this, he worked as a junior scientist at Indo-American Cancer Hospital and Research Institute in Hyderabad, India. Dr. Gowhar has over 8 years of experience in Precision and Computational Medicine and has published more than 60 research papers in journals of international repute and has authored several book chapters on Genomics. He has extensive experience in designing and deploying data analytics tools and clinical assays. Dr. Gowhar has successfully developed and commercialised genomic tests based on next generation sequencing technology of enduring value that he was instrumental in envisioning, crafting the architecture of, doing the early R&D, prototyping.
Dr Gowhar’s primary interest is in technological leadership positions that couple team-leadership with a high-octane technical hands-on involvement in machine-learning/AI models and multi-omics data sets. What distinguishes him is a technological leadership that brings together extensive, very hands-on technical ability on the one hand, and a capacity to bring together from scratch a very productive, creative, passionate and happy team across multidiscipline.
Dr Gowhar’s strength is deep hands-on expertise in both wet-lab experimentations of Molecular tumor Biology, Immunology and strong data analytics capabilities including state-of-the-art in machine-learning/data-mining algorithms and its application. In addition, he has experience in building corporate relationships, networking and convince potential business partners.

____________________________________________________________________________________________
Executive level leadership with extensive experience in drug development, clinical science, translational medicine and overseeing development strategies for early to late stage assets. Initiation and execution on Academic Alliances and Partnerships. Known for versatility and unique ability to extend skills across diverse functional roles, including strategic planning, product development, brand strategies, ability to develop trusted relationships with both internal and external partners, exceptional medical and scientific knowledge with a broad understanding of disease entities and corresponding molecular and business implications.

____________________________________________________________________________________________
Dr. Rob Monroe leads ACD’s efforts to develop diagnostic and CDx applications with the RNAscope platform. In this role, Dr. Monroe works with pathologists and researchers to identify unmet needs in biomarker testing and subsequently oversees development of RNAscope-based tests for these applications. Dr. Monroe also serves as the clinical lead in ACD’s CDx partnerships with Leica Biosystems and multiple biopharma companies for the development of CDx assays using RNAscope technology. Prior to ACD, Dr. Monroe served as Medical Director and VP Laboratory Operations for Veracyte, a diagnostics company focused on molecular cytology. Prior to Veracyte, Dr. Monroe served as Chief Medical Officer of BioImagene (acquired by Roche in 2010), a leading provider of imaging solutions for anatomic pathology, where he headed development of software applications for pathologists, including multiple FDA-cleared algorithms for immunohistochemistry. Dr. Monroe holds an MD/PhD from Harvard and is board-certified in cyto-, anatomic, and clinical pathology.from Harvard Medical School, and completed residency and fellowship training at Stanford and UCLA.

____________________________________________________________________________________________
Innovative, collaborative, and accomplished researcher with 10+ year of experience in identifying, developing, and applying innovative solutions in Biomedical and life sciences. Rigorous scientific intellect and a keen desire to generate new scientific knowledge and manage the knowledge to address unmet needs of human health and Life sciences. Energetic and detail- oriented strategic thinker with expertise in the domain of Molecular and Cellular Infection biology, cancer biology and bioinformatics.
Published in high quality journals and established expertise in animal and non animal models for bacterial infections, cancer biology and development of drug discovery tool. Proven experience in positions involving research program management and administration (e.g., strategic planning, program evaluation, program monitoring and oversight).
Demonstrated proficiency in data visualization and modeling skills such as Tableau demonstrated experience with programming and data analysis coding languages such as R (preferred), Python, SQL, Matplotlib, Pandas and python libraries. (https://github.com/anjurupesh)
Possess excellent written and oral communication skills including the ability to summarize issues. Proven ability to champion and advance cross-functional project teams Track record of success in providing strategic and technical know-how across multiple therapeutic areas, effectively collaborating and managing team performance to meet the goal and milestones.
Passionate and open to opportunities to apply my existing capabilities and innovative data analysis and computational sciences solutions to discovery and management of Life Sciences R&D processes.

____________________________________________________________________________________________
25+ years of General Management (MD, CEO, COO )at serval companies SIEMENS Gigaset Communications Gmbh.

____________________________________________________________________________________________
Dr. Gordon has worked in oncology for over 19 years and the diagnostics industry for over 10 years developing innovative diagnostic products for early cancer detection (screening tests), companion diagnostics, infectious disease identification, and monitoring. Dr. Gordon has contributed to the development of multiple FDA-approved products and understands how to get a product across the finish line. Dr. Gordon’s passion for assay development started 19 years ago when she worked at Sanofi developing cell-based screening assays for drug discovery. Dr. Gordon has hands-on experience analytically and clinically validating tests spanning genomic, proteomic and transcriptomic technologies, establishing and managing biobanks to support sample sourcing for test development, leading cancer biomarker discovery programs, and managing laboratory testing and personnel. Dr. Gordon is knowledgeable on the regulatory pathways for diagnostic tests and has experience developing these tests in accordance with CAP, CLIA, NYS-DOH, EU, and US-FDA regulations. Dr. Gordon has written regulatory submissions to support both the investigational use and marketing of diagnostic tests and has led interactions with regulatory agencies.

____________________________________________________________________________________________
Dr. Mishra (Ex-Genentech, Ex-NCI, Ex-NIH) is the Founder and CEO of Agility Pharmaceuticals. He is a serial entrepreneur who founded his first company after graduating high school, and second company during his PhD. Since then, he has accumulated a wealth of experienced by building, investing and advising numerous other companies. He is an investor/ co-founder/ board member of a few US based corporations focused on accelerating drug discovery & development, blockchain & digital health. Dr. Mishra is also founding president & CEO of American Association for Precision Medicine (AAPM) and is leading research efforts focused on preventing & curing chronic diseases; not only treating the sick but also providing knowledge/tools to individuals to live longer, healthier lives. Widely recognized as a thought leader in the field of Technology and Healthcare, Dr. Mishra has keynoted, chaired, curated and organized numerous successful international conferences. Prior to Agility Pharma, Dr. Mishra served as a scientist and Principal Investigator at Genentech, Roche. Moreover, his passion for drug development and precision medicine has led to identification of new drug targets, biomarkers, companion diagnostics and several drugs/ combinations in the clinic. His research work has been recognized globally by over 40 prestigious awards and honors. Due to his unique perspective & selfless service, he is highly respected & sought-after mentor to many. The companies that he has advised have raised multimillion dollars. Notably, he serves as a mentor and advisor to startup accelerators such as IndieBio, Health2.0’s Project Zygote and CLSI FAST program, to help grow California’s life sciences innovation ecosystem.

____________________________________________________________________________________________
After a 35 year technology career, Piyush now spends his time advising entrepreneurs and technology companies. From 2009-13, Piyush was Vice-President, Product Management at IBM with responsibility for various software product families including Information Integration, Big Data, Database & Warehousing. Piyush came to IBM via the acquisition of Exeros, where he was the founding CEO from 2004-09, growing it from inception through two rounds of financing, delivery of market leading products, acquisition of 20+ Fortune 500 customers, and the sale to IBM. He has raised about $50M in venture funds during his startup career.
Prior to Exeros, Piyush was Founder/CEO of Promeria Software, sold to Ariba during 2004. Promeria was an offshoot of LiquidPrice.com, a venture funded consumer eCommerce reverse auction site that Piyush founded in 1999 and led as CEO. From 1995-97 Piyush was Vice-President Engineering at Informix Software, with cradle to grave responsibility for several product areas.
From 1984-95, Piyush held various technical and management positions at IBM. Piyush started his IBM career at the Thomas J. Watson Research Center and left after delivering the pioneering Enterprise Information Integration Product, DataJoiner, (now called Federation Server) to the market in 1995. Early in his career, he spent three years developing Real-time Software at Schlumberger Corporation.
Piyush serves on the Board of Directors of LinguaNext, Sirion Labs, WaterLine Data and iNDx Lifecare, and the Advisory Boards of Numerify, WujiTech and ZafeSoft. He also consults for the Office of the CEO at Infosys. Past engagements include consulting at SAP and Board of Advisors at Bityota (exited to LifeLock) and SpeeDEV (exited to Kovair). Piyush is an informal advisor and mentor to numerous young entrepreneurs and startup companies. He holds a B.Tech (EE) from IIT Kanpur, a Masters (CS) from UT Austin, and an MBA from Columbia Business School.

____________________________________________________________________________________________
Mohan is the cofounder and CEO of iNDx Technology, a silicon valley based healthcare informatics company focusing on Translation Research in Oncology using AI/ML/NLP technologies.
He is a serial entrepreneur with over 30 years of experience in successfully founding, establishing and exiting high-tech and Biotech startups in Silicon Valley, raising over $50M in funding and generating over $500M in shareholder value.
Mohan has joined Stanford University Medical School as a Research Scholar to work on developing new Socio-Economic business models that leverage innovation in affordable medical technology.
Mohan was the cofounder and CEO of iNDx LifeCare, next generation Point of Care Company, Oncomdx, an innovative Cancer Diagnostics company, which specialized, in integrated diagnostics.
Mohan was the cofounder and CEO of BioImagene, a pioneer in Digital Pathology that was acquired by Roche in 2010 for $100M. He was the co-founder and CEO of Roamware Inc., a leading wireless roaming software company.
He previously held various software development and management positions with Hewlett Packard and Intel Corporation.
Mohan holds a Masters Degree in Computer Science from the Florida Institute of Technology and a Masters Degree in Electrical Engineering from Bombay University, and attended and advanced Management Program at Stanford University. He belongs to the VRCE batch of 1979

____________________________________________________________________________________________
Coming Soon

____________________________________________________________________________________________
Dr. Arun Jamkar has begun his journey as a medical student from GMC Aurangabad. Since then, he has spent three decades teaching Surgery and performing thousands of major operations in places like Grant Medical College, Mumbai and BJ Medical College, Pune. His consistent inclination towards medical research resulted in a Ph.D. in Surgical Oncology from National Institute of Virology, Pune. A significant milestone of this journey was attaining the position of Dean of BJ Medical College, Pune.
He was also fortunate to serve as the Vice Chancellor of Maharashtra University of Health Sciences, Nashik. Over the years he has helped create an ecosystem for interdisciplinary research between Science, Technology, Ayurvedic and Homeopathic Medicine. One such example of interdisciplinary collaboration would be Clinical Engineering by creating Engineers for Hospital.
He was also proud that he created a movement of teaching Bioethics in the medical curriculum through national nodal center of UNESCO chair of bioethics in Haifa in all state medical universities of India. This was possible for him because of the position of Chairman, Association of health professional universities of India
I wish to continue to serve the society by applying technology to improve the health and quality of life of the common man.

____________________________________________________________________________________________
As an actively practicing physician, a speaker and an innovator, my mission is to not just empower healthcare providers to effectively use innovation but enable them to be a “part of innovation”. ‘Dr. Darshan Gandhi is truly a thorough, caring and technically excellent physician…He has helped many of my patients with difficult problems and takes that same passion for quality patient care and creative problem solving into his leadership…” Given the complex and multi-layered health system we live in, I have engaged in several different positions, as leading Innovation/Strategy at a VC backed company, as Executive Director of Innovation/Venture Program at a large healthcare system and practicing Oncologist, within hospital systems, outpatient medical clinics, and corporations, to further that goal.
I founded the Oncology program at Methodist Charlton hospital and took it to achieve National Accreditation. Working with United Health Care as well as OptumRx National Pharmacy & Therapeutic committee, I provide insight and decision-making for drug formularies – decisions that affect 65M+ lives.
I have played an active role with Drug Contracting within US Oncology/McKesson Specialty Health. “[Dr. Gandhi’s] insights, experience and commitment to evidence-based deliberation principles have been an enormous asset to us in our formulary evaluation of new and existing drug products (particularly oncologic agents)…”
Key to providing outstanding patient care is providing resources to healthcare stakeholders. To this end:
I co-founded an online and onsite ‘ecosystem’ (HealthEntrepreneur) that fosters innovation among physicians, technologists, hospitals, and investors.
I identify problems, create business plans, prepare pitches, and raise funds for several healthcare start-ups as a trusted medical advisor and board member.
Actively involved in the community, I am also frequently called upon to write for magazines and WebMD as well as TV talk-shows.
